1. 华润医药集团简介
2016年7月,《财富》杂志全球同步发布了2016年世界500强排行榜,华润集团以营业额765.74亿美元名列第91位,排名较2015年上升24位,延续连年上升态势。
华润医药集团有限公司是华润集团根据国务院国资委“打造央企医药平台”的要求,在重组国内优势医药资源的基础上成立的大型医药研发、制造和分销企业,是华润集团旗下七大核心战略业务单元之一。2016年10月华润医药在香港联交所主板成功挂牌上市。
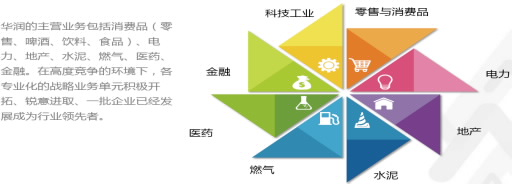
华润医药集团整体规模位居中国医药行业第二;业务领域涵盖医药制造、分销、研发等领域; 制药业务涉及天然药与中药、化学药、生物药及营养保健品等领域;拥有“999”、“双鹤”、“赛科”、“东阿”、“紫竹”、“毓婷”等中国驰名商标; 分销业务涵盖药品、医疗器械和保健产品的批发、物流配送、零售与供应链增值服务领域。
关于华润医药集团的更多信息,请浏览本公司网站http://www.crpharm.com
2. 岗位需求
1) 创新药研发副总经理
2) 研发项目管理总监
3) 研发项目质量研究总监
4) 研发项目制剂开发总监
5) 药物化学研究组长
6) 药代研究负责人
3. 创新药研发副总经理
1) 岗位描述:
a) 领导多个在研创新药项目,加速临床前候选药物及上市注册申请项目的开发;
b) 主导新研发项目删选、评价、立项;
c) 对项目过程实施科学的管理,适时调动资源加速优势项目开发进程,暂停、终止风险较大项目;
d) 招募和培训新的下级员工,实现关键岗位人员匹配,研发各环节设置有序、科学;
e) 领导团队遵守知识产权保护、安全等公司规章制度;
f) 组织有潜力在研品种国际化注册。
2) 任职资格:
a) 生物医学博士学位,在肿瘤、心血管、免疫等领域有10年或15年以上从业经验,有在国际知名公司或研发机构工作经历者优先;
b) 熟悉药物的开发过程、生物标志物的鉴定和转化医学研究等知识,精通细胞生物学、分子生物学、酶学等,具有领导开发临床前候选化合物/药物的多年实际经验;
c) 在知名期刊或会议中发表多篇文章或论文,有优秀、扎实的专业知识背景和基础;
d) 有丰富的管理经验和组织协调能力,对研发战略、发展方向有一定思考和实践;
e) 优秀的组织、沟通能力及资源协调、管理能力。
4. 研发项目管理总监
1) 岗位描述:
a) 全面监控项目从先导化合物结构优化、药理学、DMPK、CMC、安全评估(毒理学、病理学、分析/生物分析)等过程,直至IND/NDA提交;
b) 从项目全局层面协调、管理内部资源,积极识别潜在问题并给出建设性意见;
c) 组织多学科注册申报资料的编写、汇总,并从专业角度提供一定的支持;
d) 组织研究结果动态讨论与咨询:根据收集到的更新数据/结果,适时对项目研究结果和进程进行调整,以达到最佳预期结果。
2) 任职资格:
a) 博士毕业5年以上工作经验,或者硕士毕业8年以上工作经验;
b) 有相关的体外/体内、CMC、药理学,药代动力学、毒理学、生物分析等研究经验/经历;
c) 有较丰富的项目管理经验,有注册申报经验者优先;
d) 优秀的中、英文写作及口语表达能力,良好的信息收集及分析能力;
e) 人品正直、直率、敬业、有责任心、事业心强、工作细致、积极主动、严谨高效;
f) 身体健康,有一定的耐压性和吃苦精神,具有良好的心理素质、优良的沟通协调能力及职业道德和团队协作精神。
5. 研发项目质量研究总监
1) 岗位描述:
a) 领导团队从事药物研究和开发过程中有关原料药和制剂的所有质量研究工作;
b) 负责研发产品质量监察,把控项目进度、效率等;
c) 打造一流的分析研究和开发团队,招聘新员工,为新员工及现有人员提供技术指导和培养等;
d) 学习、评估国内外新技术在该领域的适用性,以提高整体研发能力;
e) 主导有潜力在研品种国际化注册中的所有质量研究部分工作。
2) 任职资格:
a) 分析化学博士学历,10年以上药物研究/开发从业经验;
b) 有在国际制药公司工作经验,5年以上管理经验;
c) 熟悉FDA、EMA、ICH、CFDA相关法规及指导原则,熟悉cGMP;
d) 优秀的中、英文写作及口语表达能力;
e) 人品正直、直率、敬业、有责任心、事业心强、工作细致、积极主动、严谨高效;
f) 身体健康,有一定的耐压性和吃苦精神,具有良好的心理素质、优良的沟通协调能力及职业道德和团队协作精神。
6. 研发项目制剂开发总监
1) 岗位描述:
a) 熟悉药物制剂开发,可领导制剂项目的研究开发过程以及临床样品制备、生产转移等工作;
b) 根据项目需求,组建新的团队并使用新技术开发新的剂型;
c) 根据公司发展需要,打造新的制剂技术平台;
d) 可有效对制剂开发过程进行管理,确保项目质量、进度、效率达成;
e) 打造一流的制剂研究和开发团队,招聘新员工,为新员工及现有人员提供技术指导和培养等;
f) 主导有潜力在研品种国际化注册中的所有制剂部分工作。
2) 任职资格:
a) 药学博士或硕士学历,有丰富的固体制剂和液体制剂开发经验和临床样品制备经验;
b) 熟悉FDA、EMA、ICH、CFDA相关法规及指导原则,熟悉cGMP;
c) 有丰富的小试到中试产品转化经验,可解决研究过程中出现的技术问题;
d) 优秀的中、英文写作及口语表达能力;
e) 人品正直、直率、敬业、有责任心、事业心强、工作细致、积极主动、严谨高效;
f) 身体健康,有一定的耐压性和吃苦精神,具有良好的心理素质、优良的沟通协调能力及职业道德和团队协作精神。
7. 药物化学研究组长
1) 岗位描述:
a) 根据项目特点及整体任务要求,制定详细、可行的研究计划/方案,并带领团队有效实施;
b) 参与化合物设计、合成路线设计,组织团队成员合成目标化合物,并对其进行评价、改进;
c) 带领团队,解决项目过程中出现的合成及药化问题,与药代、药理等同事良好技术沟通,协同促进项目顺利推动;
d) 为新员工及组内人员提供技术指导和培养等。
2)任职资格:
a) 有机化学或药物化学博士,制药企业3年以上工作经验者优先;
b) 深厚的有机合成及药物化学知识功底,精通多步合成反应,分析问题及解决问题能力突出;
c) 能够熟练检索和运用各种化学文献,熟悉化合物分离纯化、结构确证等技能;
d) 优秀的中、英文写作及口语表达能力;
e) 人品正直、直率、敬业、有责任心、事业心强、工作细致、积极主动、严谨高效;
f) 身体健康,有一定的耐压性和吃苦精神,具有良好的心理素质、优良的沟通协调能力及职业道德和团队协作精神。
8. 药代研究负责人
1) 岗位描述:
a) 带领团队成员从事生物样品处理(血浆、胆汁、尿液、粪便、植物、组织等)及检测、分析等系列工作,服务整个新药研究过程;
b) 使用HPLC、UPLC、LC-MS、LC-MS/MS等设备测定生物样品,完成新化合物体外和体内代谢研究,并对实验数据进行准确的分析和解析;
c) 用LC-MS、核磁共振和其他分析方法鉴定药物代谢产物,解析代谢路径,并形成报告;
d) 为新员工及组内人员提供技术指导和培养等;
e) 与其他同事良好技术沟通,协同促进项目顺利推动。
2)任职资格:
a) 药物分析化学等专业,博士学历,制药企业3年以上工作经验者优先;
b) 熟练使用LC/MS 、LC-MS-MS 等仪器;
c) 优秀的中、英文写作及口语表达能力,出色的沟通、交流能力;
d) 人品正直、直率、敬业、有责任心、事业心强、工作细致、积极主动、严谨高效;
e) 身体健康,有一定的耐压性和吃苦精神,具有良好的心理素质、优良的沟通协调能力及职业道德和团队协作精神。
9. 薪酬与福利
1) 员工福利:五险一金、补充医疗保险(职工、子女)、免费单身宿舍、廉租职工宿舍、免费班车、免费工作餐、年度健康体检、节日福利、生日贺礼。
2) 薪酬待遇:我们的目标是在与同行业相比较时,我们的员工拥有更具竞争力的薪水。
3) 休息休假:执行劳动法规定的休息休假制度。除双休日、国家法定假日外,公司员工还可享受年假等带薪假。
4) 培训学习:根据项目和人才培养需求,安排参加社会培训、参加国内外专业会议等,公司内部定期邀请业内知名同行举办技术交流会等。
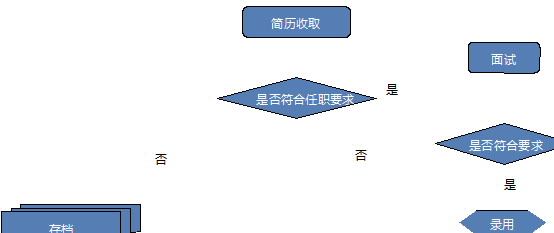
10. 招聘流程
11.联系方式
田先生:tianweixue@crpharm.com, +86-10-57985048
潘先生:panjun@crpharm.com, +86-10-57985093
12. 其他事宜
工作城市:北京
薪资面谈

CR Pharma R&D Talents Recruitment Schedule
---- Global Recruitment Febuary 2017
1. Company profile
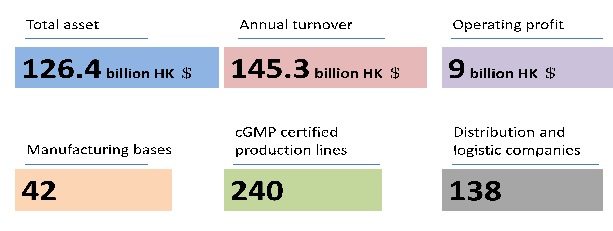
China Resources Pharmaceutical(CR Pharm)is the 2nd largest pharmaceutical company in China.(For more information please visit: www.crpharm.com)
2. China Based Jobs
1) VP of R&D
2) IND-Enabling and Regulatory/Technical Program Management Director and above
3) Director and above – Analytical R&D
4) Formulation Development Director or above
5) Principle Scientist and above of Chemistry
6) DMPK Met ID Assistant Director or above
3. VP of R&D
1) Responsibilities:
a) Lead multiple integrated projects to accelerate development of preclinical candidates.
b) Proactively contribute to the new R&D projects approval and win projects thru research proposals.
c) Execute program well and on budget.
d) Recruit and train scientists at all levels.
e) Ensure team compliance on IP protection, safety, environmental and company regulations.
2) Basic Qualifications:
a) Doctoral degree in a biomedical discipline with 10+/ 15+ years of industrial and academic experience , preferably from renown research groups and institutes, working in oncology , cardiovascular , immunology etc. Involving drug development, cancer signaling, biomarker identification, and translational research, with in-depth knowledge in cell biology, enzymology, molecular biology, and oncology models. Prior experience playing a leading role in the development of preclinical candidates/current drugs is a key asset.
b) Demonstrated leadership skill. Proven ability to direct teams of scientists and work with multi-disciplinary teams in a matrix environment.
c) Demonstrated excellent scientific skill as shown in publication of original research articles in high impact journals and in presentation of research results in scientific conferences.
d) Demonstrated ability to think strategically, design and direct original research, draw sound conclusions and communication research results to peers and management.
e) Good interpersonal and organization skills, with a good sense of prioritization of resource management.
4. IND-Enabling and Regulatory/Technical Program Management Director and above
1)Responsibilities:
a) Work in a high motivated team and very dynamic environment, you will extend your potential to limitless.
b) Scientific monitoring process of projects:RTPM will scientifically monitor all the process starting from lead optimization, pharmacology, DMPK(drug metabolism and pharmacokinetics), CMC, safety assessment(toxicology, pathology, analytical/bioanalytical), all the way through IND/CTA submission.
c) Coordinate with internal expertise to design appropriate studies to meet regulatory requirements, ultimately to meet the project needs. Stay on the top of project with continues scientific and technical support. Proactively identify potential issues and input the constructive suggestions.
d) With or without supervision, prepare the regulatory submission documents and dossier writing for analytical/bioanalytical, pharmacology, DMPK, or/and safety assessment parts. Search and prepare scientific references for project needs.
e) Dynamic study results discussion and consultation:Based on the collected updating data/results, RTPM will continually tune up the studies and process during the progress of project for the best outcome.
2) Basic Qualifications:
a) Ph.D with 5 years and up or Master with 8 years and up relevant experience in in vitro /in vivo, CMC, or pharmacology, or DMPK, or safety pharmacology, or toxicology, or bioanalytical.
b) Project manager experience(manage the preclinical program)must.
c) Biological science education background.
d) Experience in vitro or in vivo studies in research and drug discovery.
e) Regulatory background,regulatory writing experience will be a plus.
f) Excellent English and Chinese in verbal and writing,good interpersonal skill and teamwork spirit.
g) Capability to research and search information.
5. Director and above – Analytical R&D
1)Responsibilities:
a) The incumbent is to lead an analytical research and development team to conduct R&D activities from early phase, late phase through to commercial phase support for API, formulation product, stability and regulatory CMC filing, as a part of CMC development or standalone services.
b) Develop/build a top-notch analytical research and development team including planning, recruiting/hiring, coaching/mentoring, leadership training for staffs and managers, provide technical guidance and evaluate new technologies in the field to enhance capabilities.
c) Play a leading role in project management, formulation development, process development and quality assure units, prepare, review and approve quotes or proposals, supervise project review meeting by TC or on-site visits to ensure the projects meet the expected quality and within the desired timelines and budget.
2)Basic Qualifications:
a) Ph.D. degree in Analytical Chemistry or other directly related science discipline with industry experience of over 10 years in Analytical R&D/Quality Control within CMC/pharmaceutical development function; experience in large US or Europe pharmaceutical companies and a minimum of 5 years of managerial experience desired.
b) Excellent managerial experience in both people and projects being able to lead a large team of scientific staffs and senior managers and a large portfolio of CMC development and management programs.
c) Proven track record of accomplishments in the Analytical R&D and CMC development area - managing different phases of new drug development program and launching new products in major global markets desirable.
d) Must be proficient with the requirements of the FDA, EMA and sFDA cGMP regulations, ICH guidelines as well as drug development process。
e) Superb communication skills, both written and verbal, and outstanding interpersonal skills are required.
6. Formulation Development Director or above
1)Responsibilities:
a) Lead the formulation development and Clinical Trials product manufacturing groups.
b) Build new capabilities(like modified release)within the formulation, by building up team and by evaluating new technologies/ equipment.
c) Contribute to budgeting process of formulation / CT manufacturing part and ability to operate within budget by maximizing efficiencies and pioneering innovative techniques.
d) Provide technical oversight(guidance)to formulation development staff and clinical trial product manufacturing staff in both development and manufacturing projects.
e) Plan, co-ordinate, and oversee the work activities of scientific staff within the different groups. Provide technical guidance to group leaders and troubleshoot personally on the shop floor where necessary to ensure smooth progress of projects.Ensuring ongoing training programs within department to ensure continuous growth of department personnel.
2)Basic Qualifications
a) MS or Ph.D. degree in Pharmaceutics or other directly-related science discipline with sufficient experience or training in formulation development as well as clinical product manufacturing.
b) Demonstrated record of accomplishments in the formulation development of solid and liquid oral dosage forms intended for Phase I/II clinical trials.
c) Must be proficient with the requirements of the FDA/ EMEA cGMP regulations, ICH guidelines as well as drug development process.
d) Experience in handling of formulation development for late stage dosage forms upto technical transfers is required.
e) Problem solving ability and adept handling of formulation teams is also required.
f) Strong technical expertise in formulation development and CT manufacturing.
g) Strong communication skills, both written and verbal, and outstanding interpersonal skills are essential job requirements.
7. Principle Scientist and above of Chemistry
1)Responsibilities:
a) Define chemistry project team objectives, timelines, and work plans and Work closely with each head .
b) Design compounds based on SAR, and have them synthesized and evaluated.
c) Identify and solve synthetic and medicinal chemistry problems to advance the project.
d) Communicate effectively in both verbal and written form and discuss regularly with the team, and supervisor in regards to status of projects, potential issues and plans.
e) Interact closely with biology, DMPK scientists.
2)Basic Qualifications:
a) Ph.D. in organic chemistry or medicinal chemistry.
b) 3+ years of Pharm industrial experience degree is a plus.
c) Thorough knowledge of synthetic organic/medicinal chemistry, demonstrated expertise in organic synthesis, chromatographic purification methods, and compound characterization using modern analytical techniques(NMR, MS, HPLC, etc.)
d) Proven track record of scientific achievement through publications and patents.
e) Show a strong sense of responsibility and work ethic.
f) The incumbent will be required to focus on multiple issues at one time, and must have the ability to organize and direct diverse activities in a changing environment, often under time pressure.
g) Excellent oral and written communication skills both in English and Chinese.
8. DMPK Met ID Assistant Director or above
1)Responsibilities:
a) Biological sample(plasma, bile, urine, feces, plant, tissue)processing.
b) Profile biological samples using HPLC.
c) Conduct in vitro and in vivo metabolism studies.
d) Conduct LC-MS/MS analysis and interpretation.
e) Identification of drug metabolites using liquid chromatography-tandem mass spectrometry, NMR, and other analytical methodologies.
f) Elucidation of metabolic pathways.
g) Prepares scientific reports that document the analytical results and interpretation for the identification of drug metabolites.
h) Collaborate with other scientists in a team to support metabolism studies.
2)Basic Qualifications:
a) A Ph.D. degree in organic or analytical chemistry, natural product chemistry, biochemistry, pharmaceutical science or related is required.
b) 5 years’ experience in one of pharmaceutical DMPK areas.
c) Knowledge of LC/MS application in pharmacokinetics and drug metabolism.
d) Experience in LC-MS-MS method development in quantitation and metabolite profiling of biological samples is plus.
e) Experience and knowledge of drug biotransformation and identification of drug metabolites.
f) Experience in analytical method development using mass spectrometry and spectral data interpretation.
g) Good oral and written communication skills.
9. Contact Information
Mr.Tian:tianweixue@crpharm.com, +86-10-57985048
Mr. Pan:panjun@crpharm.com, +86-10-57985093
10. Other informations
Work Place:Beijing, China
Salary needs negotiation