The FDA has recently approved the following devices to be marketed. Additional items can be found on the Recently Approved Devices page.
Restylane Eyelight – P040024/S135
Restylane Eyelight is a gel implant or dermal filler that is injected in specific areas of the face to add definition or reduce the appearance of lines and wrinkles. It consists of the chemical hyaluronic acid, 1,4-butanediol diglycidyl ether(BDDE), and 0.3% of the drug lidocaine to reduce pain on injection. This approval expands the use of this product to include improving the appearance of depressed, sunken, or hollow areas under the eyes(infraorbital hollowing).
SKINVIVE by JUVÉDERM – P110033/S059
SKINVIVE by JUVÉDERM is a gel implant or dermal filler that is injected in specific areas of facial tissue to add definition or reduce the appearance of lines and wrinkles. It consists of the chemical hyaluronic acid, 1,4-butanediol diglycidyl ether(BDDE), and 0.3% of the drug lidocaine to reduce pain on injection. This approval expands the use of this product to include injections between the layers of the skin(intradermal)to improve the smoothness of the cheeks.

TOPS System – P220002
The TOPS System is a spinal implant designed to stabilize the lower spine and maintain range of motion after surgery to relieve compressed nerves(lumbar decompression surgery).
no image
SurVeil Drug-Coated Balloon – P210025
The SurVeil Drug-Coated Balloon is a drug-coated balloon used to re-open blocked or narrowed arteries in the thigh and knee due to peripheral artery disease(PAD). The balloon is coated on its outer surface with the drug paclitaxel; a drug which may help prevent the arteries from narrowing again(restenosis).

HeartSync Multifunction Disposable Single-Use AED Defibrillator Pads – P200007
HeartSync Multifunction Disposable Single-Use AED Defibrillator Pads(HeartSync Defibrillation Electrodes)are disposable electrode pads, or sensors, that can deliver an electrical shock from a defibrillator to someone whose heart has stopped beating(cardiac arrest). Together with a compatible defibrillator, these pads help detect and correct irregular heartbeats.
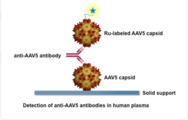
AAV5 DetectCDx – P190033
The AAV5 DetectCDx is a laboratory test that detects antibodies to the virus, adeno-associated virus serotype 5(AAV5), a virus people are commonly exposed to in the environment. This test helps doctors identify adults with severe hemophilia A who do not have pre-existing antibodies to AAV5 and who may benefit from personalized treatment with the AAV5-based gene therapy ROCTAVIAN(valoctocogene roxaparvovec-rvox).
(no image)
Optilume BPH Catheter System – P220029
The Optilume BPH Catheter System includes two catheters:the Optilume BPH Prostatic Pre-dilation Catheter and Optilume BPH Prostatic Dilation drug coated balloon(DCB)Catheter, which is coated with the drug paclitaxel. The system is used to treat an enlarged prostate, also known as benign prostatic hyperplasia(BPH), when the prostate has grown large enough to prevent the flow of urine through the tube that allows urine to pass out of the body(urethra).
CraniSeal Dural Sealant – P220014
CraniSeal Dural Sealant is an absorbable polyethylene glycol(PEG)hydrogel that is applied with an applicator over stitches(sutures). This sealant prevents cerebrospinal fluid(CSF)from leaking out of a skull(cranial)surgery incision site that includes dura mater repair. The dura mater is the tough, outermost, fibrous membrane that covers the brain and spinal cord. It lines the inner surface of the skull.

PALMAZ MULLINS XD Pulmonary Stent – P220004
The PALMAZ MULLINS XD Pulmonary Stent is a stainless-steel mesh tube that can be placed inside a narrowed pulmonary artery(blood vessel in the lung)and expanded to widen it.
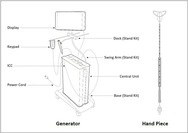
Minitouch 3.8 Era System – P230002
The Minitouch 3.8 Era System is a global endometrial ablation device used to treat heavy menstrual bleeding. These types of devices use a cold or heat energy source to destroy(thermally ablate)the lining of the uterus(endometrium)as a whole.
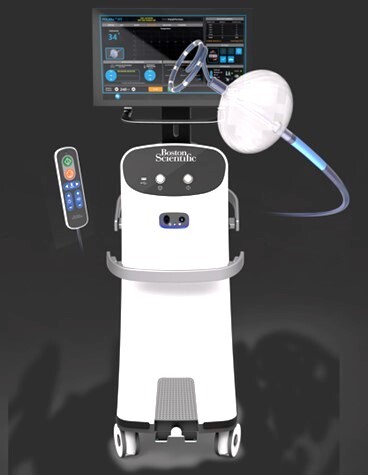
Boston Scientific Cardiac Cryoablation System – P220032
Boston Scientific Cardiac Cryoablation System and Accessories are balloon-tipped catheters that use cold energy(gas)to treat people with symptomatic recurrent paroxysmal atrial fibrillation(PAF)who do not respond to medicine(drug refractory).
(no image)
LimFlow™ System - P220025
The LimFlow system allows doctors to connect an artery in the calf to a vein near the foot to restore blood flow to the feet in patients with chronic limb threatening ischemia(also known as critical limb ischemia)that are likely to have an amputation and are not good candidates for surgical bypass. The LimFlow System consists of the following components:an arterial catheter, venous catheter, valvulotome(a surgical blade), covered stent, and delivery system. The covered stent is a small hollow tube made of metal(nitinol)and covered in a polymer film. The stent and delivery system contains metallic bands that can be visualized under fluoroscopy imaging.