|
|
|
| SARS-CoV-2 structure informs clinical trials | Progress in gene therapies, immunotherapies |
|
| 2020/8/8 18:53:49 | 浏览:2199 | 评论:0 |
|
|
|
|
 |
| |
| |
|
|
|
TOP STORIES
Study analyzes how SARS-CoV-2 undermines immune defenses
A new research study used whole-genome sequencing to analyze the genetic profile of patients with the SARS-CoV-2 virus to gain more insight into how it turns the body's own immune system against itself. The findings could help researchers develop drugs to target the virus, according to an August 3 report in Nature Medicine.
|
|
 |
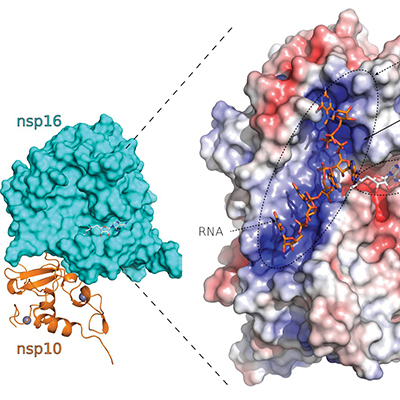
Researchers further define nonstructural protein targets of SARS-CoV-2
The crystal structure of nonstructural protein 16 of SARS-CoV-2, which plays a role in viral RNA capping to mimic host messenger RNA, reveals specific rational design targets that could be used to develop effective therapies against SARS-CoV-2 and other coronaviruses. The research was published in Nature Communications on July 24.
|
|
 | |
BIOPROCESSING
Researchers develop mouse model of COVID-19 infection
Researchers have generated a strain of SARS-CoV-2 that can infect mice and used it to produce a new mouse model of infection to help facilitate testing of COVID-19 vaccine candidates and therapies. The research article was published in Science on July 30.
|
|
 | |
CANCER & DISEASE RESEARCH Sponsored by Beckman Coulter
Neutrolis develops new COVID-19 treatment
Neutrolis has developed a new class of COVID-19 therapy, a DNASEIL3 enzyme analog that has the potential to clear neutrophil extracellular traps for severe cases of COVID-19.
|
|
 |
|
CELL BIOLOGY
Study analyzes how SARS-CoV-2 undermines immune defenses
A new research study used whole-genome sequencing to analyze the genetic profile of patients with the SARS-CoV-2 virus to gain more insight into how it turns the body's own immune system against itself. The findings could help researchers develop drugs to target the virus, according to an August 3 report in Nature Medicine.
|
|
 |
Researchers further define nonstructural protein targets of SARS-CoV-2
The crystal structure of nonstructural protein 16 of SARS-CoV-2, which plays a role in viral RNA capping to mimic host messenger RNA, reveals specific rational design targets that could be used to develop effective therapies against SARS-CoV-2 and other coronaviruses. The research was published in Nature Communications on July 24.
|
|
 |
|
DRUG DISCOVERY & DEVELOPMENT
New COVID-19 vaccine candidate leverages earlier research
Could vaccine platforms developed for previous coronaviruses be converted to address the current COVID-19 pandemic? An August 5 report in Nature described how researchers leveraged previous vaccine research to develop mRNA-1273, a new SARS-CoV-2 vaccine candidate.
|
|
 |
R&D Alliance begin I-Spy COVID trial
A collaborative research effort that includes the U.S. government and leading pharmaceutical companies has launched a clinical trial to investigate a series of therapeutics to treat patients who are severely ill with the novel coronavirus disease.
|
|
 |
Sanofi, GSK get $2.1B for COVID-19 vaccine
The U.S. government will provide up to $2.1 billion to Sanofi and GlaxoSmithKline(GSK)for the development and manufacturing of a recombinant protein-based COVID-19 vaccine.
|
|
 |
|
GENOMICS Sponsored by Nvidia
Bio-Rad's Q2 revenue drops
Bio-Rad Laboratories reported that the COVID-19 pandemic negatively impacted its revenue for the second quarter as well as for the first half of the year.
|
|
 |
|
IMMUNOLOGY Sponsored by Beckman Coulter
PROTEOMICS
NanoString launches readout technology for NGS
NanoString Technologies has released Cancer Transcriptome Atlas, a new product based on the company's GeoMx digital spatial profiler platform that's optimized for readout on Illumina's next-generation sequencing(NGS)technology.
|
|
 |
|  |
 Insights Insights
Exclusive content for Science Advisory Board members on trends and outcomes that are influencing the future of scientific research. |
| |
 Communities Communities
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities. |
| |
 Rewards Rewards
Earn points for contributing to research. Redeem your points for merchandise, travel, or even to help your favorite charity. |
| | |
| |
|
| | |
Dear Science Advisory Board Member,
As more molecular coronavirus research becomes published, we are getting a better view of the SARS-CoV-2 virus and its pathogenicity. Particularly, how the virus leverages nonstructural proteins to undermine host immune defenses and how blocking these proteins may effectively stop the virus in its tracks. More detailed molecu lar views of these accessory proteins will guide structure-based drug design and bring us one step closer to beating this pandemic.
As an example, researchers from the U.S. National Institutes of Health(NIH)and other academic institutions in the U.S. described how they rapidly developed a SARS-CoV-2 vaccine candidate based on the genetic sequences of the virus and previous work with messenger RNA(mRNA)vaccine delivery systems.
This vaccine candidate, mRNA-1273, is being jointly developed by the NIH and Moderna and is part of the agency's large-scale clinical trial protocol, Accelerating COVID-19 Therapeutic Interventions and Vaccines(ACTIV). The program includes both vaccines and treatments, and the NIH announced that it has launched phase II studies for the investigational monoclonal antibody Ly-CoV555 developed by Eli Lilly and AbCellera.
Other contenders, like Johnson & Johnson's Ad-26 and Inovio's Ino-4800, are posting positive preclinical results and their developers expect to begin late-stage clinical testing soon. Funding through the U.S. government's Operation Warp Speed program is still being allocated to new vaccine candidates, such as a recombinant protein-based vaccine being developed by Sanofi and GlaxoSmithKline.
Aside from COVID-19 developments, several notable advances in immunotherapies and gene therapies were announced this week. Bio-Rad won an important patent infringement appeal over 10X Genomics, which gives it an edge in the gene expression products market. Meanwhile, Omega Therapeutics is advancing its epigenomic programming platform in clinical trials, and SAB Therapeutics has begun testing a seasonal flu immunotherapy.
Stay healthy and safe.  Samantha Black, PhD Samantha Black, PhDThe Science Advisory Board Editor editor@scienceboard.net | | |
|
|
|

















