
TheraBionic P1 – H220001
The TheraBionic P1 System is a handheld, battery-operated, radiofrequency(RF)electromagnetic field(EMF)generator that includes an antenna on a cable that attaches to the generator. The EMF frequencies may stop cancer cells from dividing and making more cancer cells.
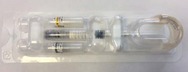
Belotero Balance®(+)- P090016/S050
Belotero Balance®(+)is a prescription gel implant or dermal filler that is injected to improve the appearance of depressed, sunken, or hollow areas under the eyes. The depression under the eyes is called infraorbital hollowing. Belotero Balance®(+)comes in a blister pack containing 1-mL of sterile gel prefilled in a glass syringe and packaged with two sterile needles and two patient record labels. This approval expands the use of this product for infraorbital hollowing. It was previously approved for moderate to severe facial wrinkles and folds.

CRCdx® RAS Mutation Detection Kit – P220005
The CRCdx® RAS Mutation Detection Kit is a laboratory test designed to detect changes in the genetic material in tumor tissue samples from a person with colorectal cancer. The results help doctors determine if a person is a good candidate for treatment with the drug, panitumumab. The drug is also known by its brand name, Vectibix. The test detects certain mutations in RAS genes in tumor samples from a person with colorectal cancer. If the test finds a mutation in the colorectal cancer tissue, then panitumumab is not recommended. If the test does not detect a mutation, then panitumumab may be an appropriate treatment.
Oncomine™ Dx Target Test – P160045/S025
The Oncomine™ Dx Target Test is a laboratory test designed to detect genetic changes in tumor tissue samples from a person with certain type of cancers. This approval expands the use of the test to identify a certain mutation in anaplastic thyroid cancer. People whose anaplastic thyroid cancer has a certain change(specifically BRAF V600E mutation), detected by the test may benefit from personalized treatment with TAFINLAR® in combination with MEKINIST®. The test was previously approved to detect certain genetic changes in tissue samples from people with non-small cell lung cancer, cholangiocarcinoma(bile duct cancer), thyroid cancer, and medullary thyroid cancer.

FoundationOne Liquid CDx(F1 Liquid CDx)– P190032/S011
The FoundationOne Liquid CDx is a laboratory test that detects a number of mutations in circulating cell-free DNA(cfDNA). This test helps doctors identify patients who may benefit from specific FDA-approved treatments. This approval expands the indications for use of the FoundationOne Liquid CDx to include testing people with non-small cell lung cancer who may have a specific genetic mutation called BRAF V600E in their tumors. Identifying whether a person with non-small cell lung cancer has BRAF V600E may help determine if they will benefit from treatment with BRAFTOVI(encorafenib)in combination with MEKTOVI(binimetinib).
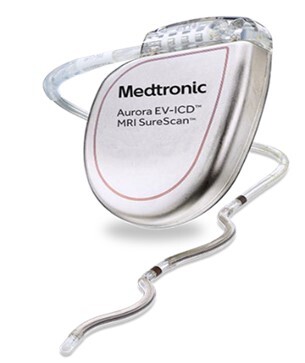
Aurora EV-ICD System – P220012
The Aurora EV-ICD System is used together to monitor the heart and provide therapy for life-threatening arrhythmias. The System is comprised of the Aurora EV-ICD MRI SureScan Model DVEA3E4 extravascular implantable cardioverter defibrillator(ICD)and the Epsila EV MRI SureScan Model EV2401 extravascular lead. The Aurora EV-ICD MRI SureScan single chamber ICD is a multiprogrammable cardiac device. The Epsila EV lead is a shaped, passive fixation, extravascular quadripolar lead.

RHA 3 Dermal Filler – P170002/S030
RHA 3 is a gel implant or dermal filler that is injected in specific areas of facial tissue to add definition or reduce the appearance of lines and wrinkles. It consists of the chemical hyaluronic acid, 1,4-butanediol diglycidyl ether(BDDE), and 0.3% of the drug lidocaine to reduce pain on injection. This approval expands the use of this product to include injections into the lips to augment the fullness of the lips.
Alinity m HR HPV for use on the Alinity m System – P230003
The Alinity m HR HPV is a laboratory test used to detect human papillomavirus(HPV)genetic material, or viral DNA, in samples taken from the lower part of a patient’s uterus(cervix). The test can also identify certain virus types(genotypes)that increase the risk of someone developing cervical cancer. The test is designed for use on the Alinity m System, which runs the assay and analyzes the results. The test can evaluate cervical samples stored in either ThinPrep or SurePath preservative fluid.
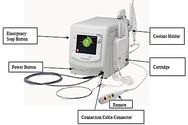
Paradise Ultrasound Renal Denervation System – P220023
Paradise Ultrasound Renal Denervation System is intended to reduce a person’s blood pressure by using ultrasound energy to disrupt nervous system signals to the kidneys. The device is intended for use to reduce blood pressure.

FoundationOne CDx(F1CDx)– P170019/S048
FoundationOne CDx is a laboratory test designed to detect genetic variations in 324 genes in addition to select gene rearrangements and other biomarkers in the genomic makeup of a tumor. FoundationOne CDx is a companion diagnostic that was previously approved for the detection of genetic mutations in people who may benefit from FDA-approved therapies for non-small cell lung cancer, melanoma, breast cancer, colorectal cancer, cholangiocarcinoma, prostate cancer, ovarian cancer, and solid tumors. This approval expands the indications for use of the FoundationOne CDx test to include testing people with breast cancer that is spreading within the breast(locally advanced)or to other areas of the body(metastatic)for changes(alterations)to the PIK3CA/ AKT1/PTEN genes. Identifying solid tumors with PIK3CA/ AKT1/PTEN alterations will help identify people who may benefit from personalized treatment with TRUQAP(capivasertib)in combination with FASLODEX(fulvestrant).

restor3d Total Talus Replacement – H230003
The restor3d Total Talus Replacement implant is a 3D printed and polished implant designed and made individually for each patient using data from computed tomography(CT)scan. The restor3d Total Talus Replacement implant is intended to reduce pain, increase physical function and maintain range of motion by avoiding limb loss(amputation)or loss of joint mobility(fusion). The restor3d Total Talus Replacement implant is designed to match the patient’s specific anatomy and is additively manufactured from a medical grade cobalt chromium metal alloy. The device has optional soft tissue attachment sites to allow ligament attachment as needed.


Duo Venous Stent System – P230021
The Duo Venous Stent System is intended to treat narrowing of or reduced blood flow through the deep veins in the groin(iliofemoral veins). The Duo Venous Stent System has two parts:a small hollow tube(stent)made of a metal called nitinol and a delivery catheter. The Duo Venous Stent System includes two types of stents, the Duo Hybrid Stent and the Duo Extend Stent.

















