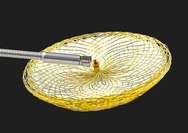
The Occlutech ASD Occluder is a permanent implant intended to treat an atrial septal defect (ASD). The device has two umbrella-shaped flexible discs with a connection in the middle. When the device is carefully positioned in the ASD, the two discs cover both sides of the atrial septum, closing the hole. After a few months, the device will be covered by a thin layer of tissue.

TZ Medical Multi-Function Defibrillation Electrodes and Adaptors monitor the heart’s rhythm and deliver a pacing or electrical shock from a defibrillator(a device that applies an electric charge to the heart to restore a normal heartbeat)to a person with an arrhythmia.
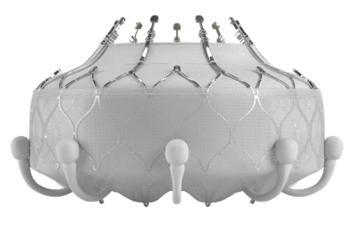
The Edwards EVOQUE Tricuspid Valve Replacement System mainly includes an artificial tricuspid valve(called the EVOQUE valve)and a delivery catheter. The EVOQUE valve is made of cow tissue attached to a self-expanding metal(nickel-titanium)frame for support. It can be used to replace the tricuspid valve of the heart without open-heart surgery. The tricuspid valve prevents backflow of blood into the right atrium(upper right chamber of the heart)from the right ventricle(lower right chamber of the heart).

Boston Scientific’s WaveWriter Spinal Cord Stimulation(SCS)Systems are implanted(placed into the body)to help reduce pain that is chronic(long-term)and intractable(difficult to manage)in the legs, arms, or trunk. This supplement expands the Indications for Use to include treatment of low back and leg pain that is difficult to manage without prior back surgery. |

The XACT Carotid Stent System is intended to be used to reopen narrowed parts of the carotid arteries in the neck that supply blood to the brain. The system consists of a delivery catheter and a self-expanding metal stent made of nitinol.
This approval expands the indications for the XACT Carotid Stent System to be used during a Transcarotid Artery Revascularization(TCAR)procedure, in which a device is placed in the neck instead of in the thigh in combination with the ENROUTE Transcarotid Neuroprotection System(ENROUTE NPS). The ENROUTE NPS temporarily reverses blood flow at the treatment area to redirect pieces that may become loose during the procedure from potentially traveling to the brain.
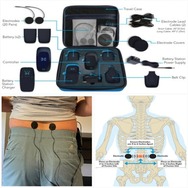
The Xstim Spine Fusion Stimulator is a bone growth stimulator(BGS)device intended be used on the lower back. The device components include a controller for creating signals, a belt clip, a rechargeable lithium-ion battery pack and charging unit, controller cables, hydrogel electrode pads that stick to the skin, optional electrode covers, including some for use only when showering, and a carry case.
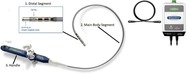
The INTELLANAV STABLEPOINT Ablation Catheter uses radiofrequency(RF)energy applied by the catheter tip to destroy heart tissue cells that are causing heart rhythm problems(arrhythmias).
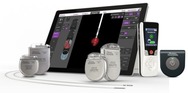
The Vercise PC, Vercise Gevia, and Vercise Genus Deep Brain Stimulation(DBS)Systems are implantable devices that deliver low-intensity electrical pulses to nerve centers in the brain. This supplement expands the Indications for Use to include an option of two-sided stimulation for people who are diagnosed with essential tremor not adequately controlled by medication and where the tremor creates a significant inability to function. |

The AGENT Paclitaxel-Coated Balloon Catheter(Agent DCB)uses a narrow tube with a balloon on the end to reopen arteries that supply blood to the heart and are blocked or narrowed due to coronary artery disease(CAD). The balloon is coated on its outer surface with the drug paclitaxel, which may help prevent the arteries from narrowing again(restenosis).
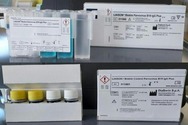
The LIAISON Biotrin Parvovirus B19 IgG Plus is a laboratory test used to detect human parvovirus IgG antibodies against the parvovirus B19 antigen in the blood.

The TriClip G4 System is used to treat tricuspid regurgitation(leaky tricuspid valve)often caused by an enlarged heart or damaged tricuspid valve leaflets, without open-heart surgery. |

ColoSense is a laboratory test that measures ribonucleic acid(RNA)and hemoglobin(blood)in human stool that is not visible to the eye(fecal occult blood), which may indicate the presence of colorectal cancer or of advanced adenomas(growths on the lining of the colon or rectum). The ColoSense Collection Kit consists of a container for collection of stool and a separate sampler for collection of stool for hemoglobin testing(only one stool is required to obtain both samples). Both samples are required to obtain a ColoSense result. Additionally, bowel prep or following dietary or medication restrictions to complete the test is not necessary.

















